-40%
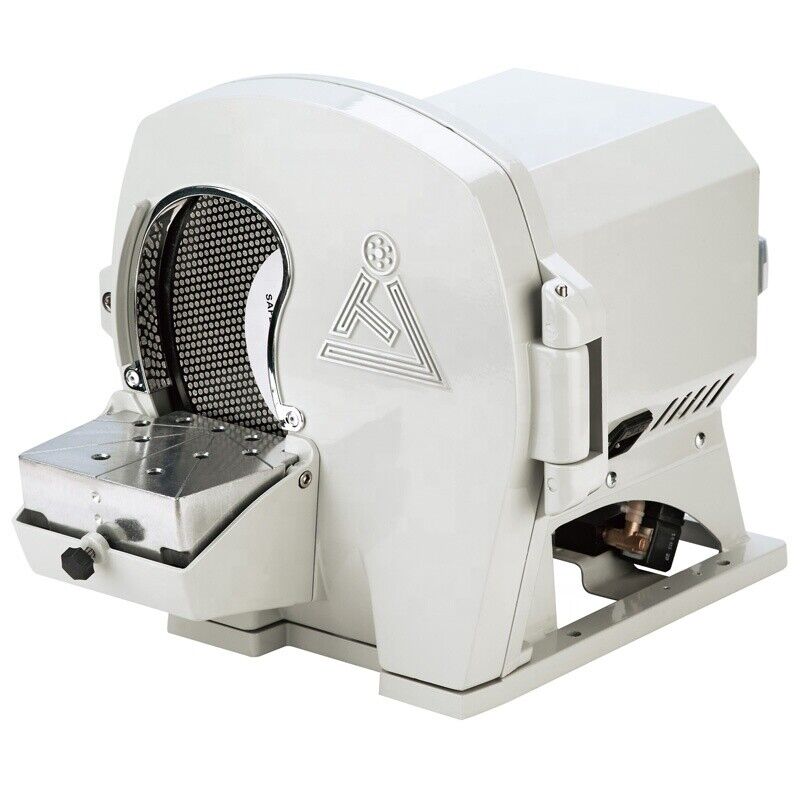
Denshine Dental Model Arch Trimmer Trimming sides of plaster models Lab Dentist
$ 173.71
- Description
- Size Guide
Description
Dental Model Arch Trimmer Trimming sides of plaster models Lab Dentist FDADescription:
Model: 370225 /370226
This Model Arch Trimmer equipped with high-quality motor, speed uniform, simple operation and rugged durability.
Technical Data:
Power Supply:220V/110V 50/60Hz
Power Consumption:150W
Motor rotational speed:2,800rpm
Grinding head:C 625 M06
Optional Accessories:Dust Extractor
Dimensions:25cm×21cm×26cm (H W D)
Weight:8.5kg
Package Included:
1×Machine
1×Manual
1×Power Cord
1×
Fuse
*Payment
1.We accept PayPal only. We will ship out the item after receiving cleared payment.
2.Please make sure you PayPal account is valid prior bidding.
3.Great appreciate for prompt payment. Unpaid item will be filed without receiving payment in 7 days after listing ended.
*Shipping
1.After full payment is received and cleared, we will ship it ASAP .
2.A complete and correct shipping address is essential.
3.Generally, mor than 200$,Expedited shipping !!!
*Return Policy
All items are with 2 Years warranty .
we accept returns, contact us within 60 days from delivery date and we will give you a full refund or exchange.
Only unused, undamaged and original condition item can be qualified for a return.
Buyer is responsible for return shipping fee.
*FDA declaration
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(
The seller Name: Lily Lee, City: Beijing ,State: Beijing,Country: China Telephone number:15001145569)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health.
*Contact Us
Any questions, please contact us through eBay message, We will reply within 1 business days (public holidays not included).
Our working time: Monday - Friday 9:00 AM - 5:30PM (Beijing)